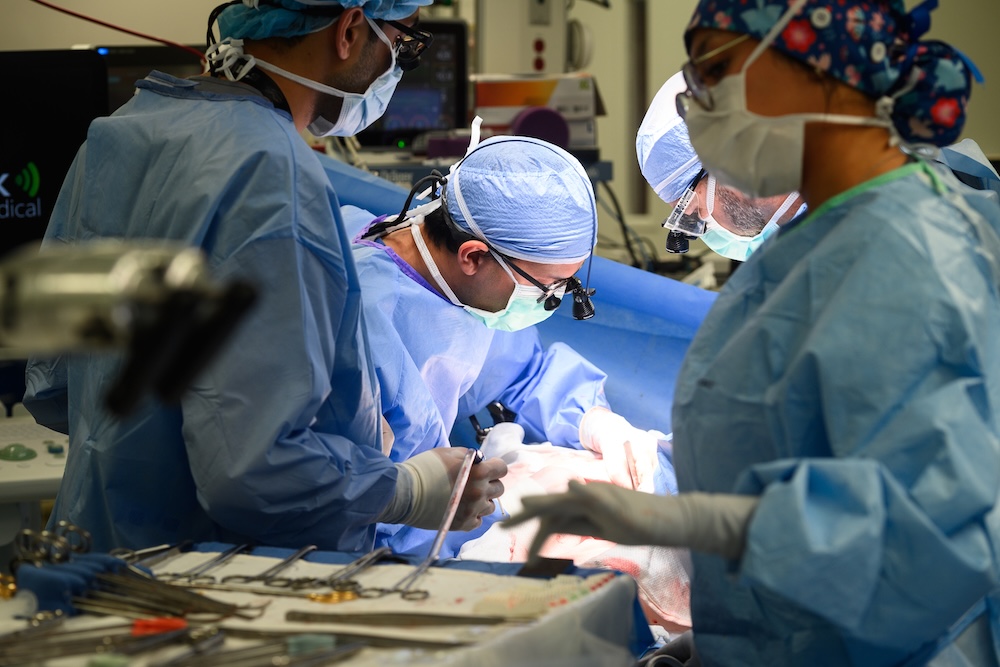
Early experience of simultaneous sleeve gastrectomy in living donor liver transplant recipients with obesity – a pilot study
Highlights Abstract Background: Increasing obesity and metabolic dysfunction-associated steatohepatitis (MASH) cirrhosis present challenges in liver transplantation. Objectives: While simultaneous sleeve gastrectomy (SG) has been described in the deceased donor liver transplantation (DDLT) […]
Why Some Livers Can’t Recover After Alcohol Abstinence
By Eugenia Tsai, MD A recent Nature Communications study by Kalsotra and colleagues offers new molecular insight into why patients with alcohol-associated liver disease (ALD)—particularly those with severe alcohol-associated hepatitis (sAH)—often fail to regain […]
University Health Transplant Institute | Trailblazing Innovations in Living Donor Liver Transplants
The Hospital That Says Yes: Inside the Nation’s Top-Ranked Liver Transplant Institute

For Melroy Koehler, transplant morning arrived with a surprise: his own bubble parade. He grinned as nurses lined the corridor and blew bubbles—a lighthearted send-off before two, back-to-back, life-changing operations. […]
The accuracy of FibroScan, FIB-4, and nonalcoholic fatty liver disease fibrosis score in predicting biopsy-defined fibrosis and steatosis across all fibrosis stages in patients with metabolic dysfunction associated steatotic liver disease
The accuracy of FibroScan, FIB-4, and nonalcoholic fatty liver disease fibrosis score in predicting biopsy-defined fibrosis and steatosis across all fibrosis stages in patients with metabolic dysfunction associated steatotic liver disease. Thallapureddy K, Twitchell D, Ott K, Pedicone LD, Owo C, Kumar N, Gelfond J, Shankar N, Goros M, Kwok D, Liles A, Ozguc F,…
Bemnifosbuvir, Ruzasvir Regimen Meets Safety, SVR Primary Endpoints in Phase 2 HCV Trial
Key Takeaways The phase 2 study of a bemnifosbuvir and ruzasvir regimen for the treatment of HCV met both primary endpoints for safety and SVR 12 weeks post-treatment. Atea Pharmaceuticals […]
Spanish Community for KVDA-TV: Liver Cancer Awareness Month
Fabian Rodas Ochoa, MD, transplant hepatologist and clinical assistant professor of medicine at UT Health San Antonio, was interviewed by KVDA-TV (Telemundo 60 San Antonio) and spoke about liver cancer, […]
Octubre es el Mes de Concientización Sobre el Cáncer de Hígado
October is Liver Cancer Awareness Month
Survodutide may have ‘positive effects on patient’s liver status’ in MASH cirrhosis
Survodutide has an “acceptable safety profile” in patients with metabolic dysfunction-associated steatohepatitis with compensated and decompensated cirrhosis, with no dose reduction needed based on pharmacokinetics, according to data. “There is a large unmet […]